


The science dealing with crystal formation and shapes is Crystallography
On the display you find a few real crystals, cut stones, and drawings of a few of the forms in which minerals and organic substances may crystallize. Of course, they are rarely so regular in nature, because so many things can disturb the slow process of crystallization: temperature, impurities, presence of other solids, movements, pressure and so on.
Why most molecules may crystallize?
On the surface of molecules, clouds of electrons are often irregular depending of the nature of the various atom types present. Some places are more negative and others more positive. Therefore molecules behave like small magnets and tend to adhere together smoothly taking the most stable positions. Molecules which are not magnet can not normally crystallize, examples: helium, neon, argon.
On the first scheme is shown the way that foreign molecules can disturb the formation of crystals. The more the foreign molecules are different from the crzstallizing molecules, the greater the disturbance.
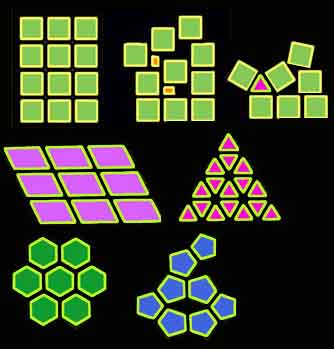
All kinds of crystal symmetries may exist depending on the shape and polarity of molecules. A pentagonal symmetry may not exist in normal crystals since it is impossible to arrange the units in a regular pattern.
Through knowledge of bond lengths, shapes of simple molecules may be approximated. The form of more complex structures may be found by taking an x-ray of their crystals (if a crystalline form can be obtained) giving the exact 3D positions of the atoms in the crystal unit.



Two nicely crystallized minerals as found in nature: Island Spar (calcite) and Fluorite.
Quartz from the Alps